Abstract
Background
Axi-cel is approved for the treatment of relapsed/refractory diffuse large B-cell lymphoma since 2017 and has demonstrated efficacy and durability but with significant side effects, namely cytokine release syndrome (CRS) and neurotoxicity (ICANS). In patients receiving axi-cel for aggressive lymphomas, any grade ICANS occurs at a rate of 50% and grade 3/4 occurs about 35% of the time. The mechanism underlying ICANS remains unclear. The current working hypothesis is that endothelial injury caused by the disease and lymphodepleting chemotherapy leads to breakdown of the blood-brain-barrier. Following activation of axi-cel, cytokines are trafficked into the central nervous system leading to local production of cytokines and the clinical syndrome observed. ICANS is treated with systemic steroids with good CNS penetration. Statins have been shown to stabilize the endothelium and have anti-inflammatory effects. We are currently evaluating the safety and feasibility of administering simvastatin to stabilize the endothelium in addition to dexamethasone delivered intrathecally to prevent the occurrence and decrease the severity of neurotoxicity.
Methods:
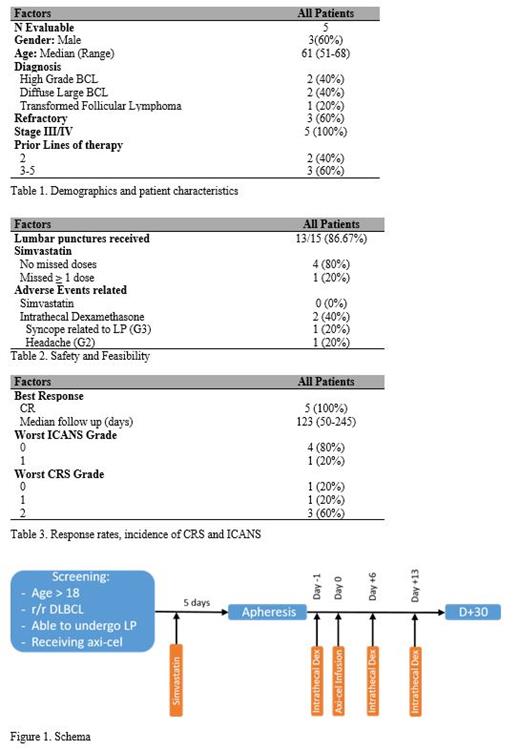
This is a feasibility study combining simvastatin 40 mg orally, daily, starting at least 5 days prior to apheresis in addition to dexamethasone delivered intrathecally on days -1, +6, +13 in relation to axi-cel. A schema of the trial is presented in figure 1. This is registered on Clinicaltrials.gov number (NCT04514029).
Results:
We have accrued 6/20 planned patients with r/r DLBCL who received axi-cel per institutional guidelines. One passed away prior to apheresis due to disease progression and was non-evaluable. Table 1 shows the patient characteristics. With a median follow up of 123 days (50-245), all patients achieved a complete response and remained alive. Table 2 summarizes the feasibility, safety, and dose-density received out of prescribed. None of the 5 patients had grade 3 or 4 ICANS and only 1 patient had grade 1 neurotoxicity (Table 3). A cytokine analysis and markers of endothelial and neuronal activation is underway.
Discussion:
In a preliminary analysis, simvastatin and intrathecal dexamethasone appears to be feasible to administer, safe and efficacious in a population of patients receiving axi-cel for relapsed and refractory DLBCL. There appears to be a signal in abrogating neurotoxicity without any effect on the efficacy of axi-cel. This will allow for a safer delivery of axi-cel and improve access to this treatment. This study is ongoing.
Bachanova: FATE: Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; KaryoPharma: Membership on an entity's Board of Directors or advisory committees; Gamida Cell: Membership on an entity's Board of Directors or advisory committees, Research Funding. Weisdorf: Fate Therapeutics: Research Funding; Incyte: Research Funding. Miller: Wugen: Membership on an entity's Board of Directors or advisory committees; GT Biopharma: Consultancy, Patents & Royalties, Research Funding; Vycellix: Consultancy; ONK Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Magenta: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Fate Therapeutics, Inc: Consultancy, Patents & Royalties, Research Funding. Janakiram: FATE, Nektar Therapeutics: Research Funding; Bristol Meyer Squibb, Kyowa Kirin, ADCT Therapeutics: Honoraria.
Simvastatin is an HMG-CoA reductase inhibitor approved for hyperlipidemia and cardiovascular health.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal